WHAT IS WATER FOR INJECTION ?
GRADE WATERS AND PHARMACOPOEIAS
Pharmaceutical Waters
Only a few types of pharmaceutical water have USP designation and are the subjects of official monographs in the current US Pharmacopeia. However, the same document says that “Although there are no absolute microbial standards for water (other than water intended to be sterile), the cGMP regulations require that appropriate specifications be established and monitored”. “(…) i.e. water used to formulate a product should contain no organism capable of growing in the product. (…)”.
This is why, at BRAM-COR, each water treatment system is truly customized and oriented to a specific pharmaceutical plant, line and product. The ISPE document (ISPE BASELINE© GUIDE, Water and steam Systems) explains what water can be used in the development, manufacture and preparation of pharmaceuticals. These waters fall into two categories:
1. COMPENDIAL WATER
2. NON-COMPENDIAL WATER
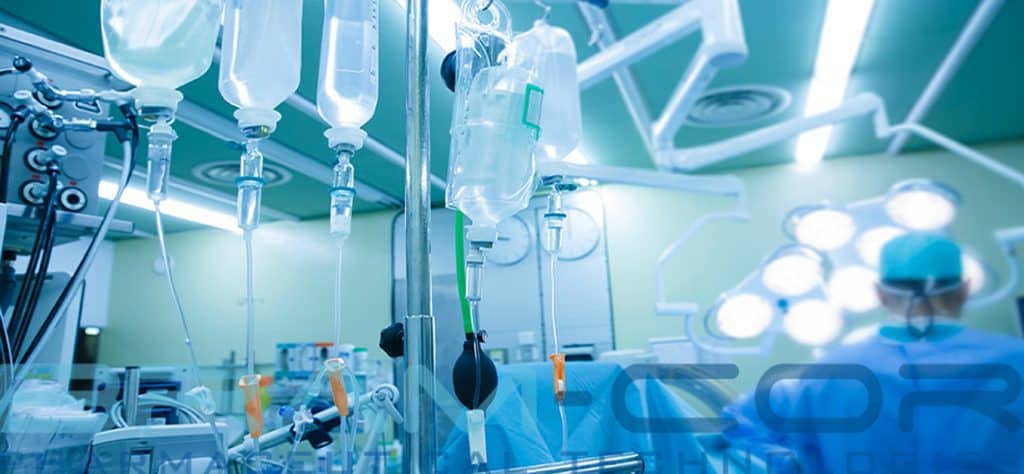
IV (infusion) bags and bottles hanging on poles during a real surgery
What Water for Injection and Purified Water mean for pharmaceutical industry
Water is employed as ingredient and solvent in many processes, formulations and products. Therefore, International pharmacopoeias (USP, Ph.Eur., JP) define as Water For Injection (WFI) only the compendial water used as an excipient to parenteral solutions. So, ISPE Glossary distinguishes:
– Water for Injection in bulk: “Water for the preparation of medicines for parenteral administration when water is used as a vehicle”;
– Sterilized Water for Injection: ” (Water) for dissolving or diluting substances or preparations for parenteral administration before use”.
Non-sterile medicinal products Minimum acceptable quality of water* Vaccines for non-parenteral use (PW) Purified Water* Oral Preparations (PW) Purified Water Nebuliser Solutions (PW) Purified Water** Cutaneous Preparations (PW) Purified Water*** Nasal/Ear Preparations (PW) Purified Water Rectal/Vaginal Preparations (PW) Purified Water
Source: EMA/CHMP/CVMP/QWP/496873/2018. Release: 13 November 2018. Note that the finished water meets all the chemical requirements for PW (Purified Water). And, with an additional bacterial endotoxin parameter, the water meets the specific WFI character. Since microorganisms that are prone to spread in the water produce endotoxins, the equipment and the procedures used by the production lines to purify, store and distribute WFI must prevent the build-up of endotoxin and minimize microbial contamination in the system.
Sterile Medicinal Products Minimum acceptable quality of water* Biologics (including vaccines and ATMP) (WFI) Water for Injection Parenteral (WFI) Water for Injection Ophthalmic (excluding ATMP) (PW) Purified Water Haemofiltration Solutions / Haemodiafiltration Solutions (WFI) Water for Injection Peritoneal Dialysis Solutions (WFI) Water for Injection Irrigation Solutions (WFI) Water for Injection Nasal/Ear Preparations (PW) Purified Water Cutaneous Preparations (PW) Purified Water
Source: EMA/CHMP/CVMP/QWP/496873/2018. Release: 13 November 2018. *Note that WFI is in any case recommended in order to ensure the vaccines’ safety and product quality. ** In certain disease states, medicinal products are required to be sterile and non-pyrogenic. In such cases, WFI should be used. ***For some products, such as veterinary teat dips, it may be acceptable to use potable water where justified and authorised.
A. PHARMACEUTICAL COMPENDIAL WATERS
Compendial waters in ISPE examples are:
1. Purified Water (PW), as defined by USP / Ph.Eur. monographs.
Purified Water is classified for pharmaceutical use as an excipient in the production of non-parenteral preparations and, in specific, for pharmaceutical preparations/tests and assays, for which water is indicated, unless otherwise specified (see related USP, Ph.Eur. and JP pharmacopeia for reference).
Therefore, drinking water is the minimal source of feed water to produce PW, through deionization, distillation, ion exchange, reverse osmosis, filtration or other appropriate procedures. PW is also a feedstock in the WFI and pure steam preparations.

Bram-Cor Reverse Osmosis System in a water treatment room, with PW Storage tank and PW Loop
Sterile Purified Water, as defined by USP, is PW packaged and rendered sterile; it is used in applications requiring Purified Water, where access to a validated Purified Water System is not serviceable (small q.ty needed, for example).
PURIFIED WATER IN BULK* | ||
| PHISICAL / CHEMICAL | Ph. Eur. | USP |
| Appearance | Colorless, clear | Not defined |
| Conductivity | ≤ 4.3 μS/cm@20°C | ≤ 1.3 μS/cm@25°C |
| TOC | ≤ 0.5 mg/l | ≤ 0.50 mg/L |
| Nitrates NO₃ | ≤ 0.2 ppm | Not defined |
| MICROBIOLOGICAL | Ph. Eur. | USP |
| Bacterial count | ≤ 100 CFU/ml | ≤ 100 CFU/ml |
| Bacterial endotoxins | < 0.25 IU/ml | – |
*data can change without notice
2. Water for Injection (WFI) as defined by USP / Ph.Eur. monographs.
Water for Injection is classified for pharmaceutical purposes as an excipient in the production of parenteral preparations and in other pharmaceutical preparations where the endotoxin content must be verified (see related USP, EP and JP pharmacopeia for reference).
Consequently, deionized water (that may be pre-treated for subsequent distillation or appropriate process) is the minimal source of feed water to produce WFI. In addition to the PW specifications, WFI adds the test for Bacterial endotoxins and complies with all requirements (except for Labeling) for the packaged water know as “Sterile Purified Water” (see USP monograph).
Sterile Water for Injection, as defined by USP. It is WFI packaged and sterilized; it is used in applications requiring Water for Injection, where access to a validated WFI System is not serviceable (small q.ty needed, for example).
Bacteriostatic Water for Injection, as defined by USP. It is WFI with addition of one or more antimicrobial preservatives; it is used as a diluent in the preparation of parenteral solutions (especially for multi-dose products that allow repeated withdrawals).
WATER FOR INJECTION IN BULK* | ||
| PHISICAL / CHEMICAL | Ph. Eur. | USP |
| Appearance | Colorless, clear | Not defined |
| Conductivity | ≤ 1.1 μS/cm@20°C | ≤ 1.3 μS/cm @25°C |
| TOC | ≤ 0.5 mg/L | ≤ 0.50 mg/L |
| Nitrates NO₃ | ≤ 0.2 ppm | Not defined |
| Aluminium | ≤ 10 ppb | Not defined |
| MICROBIOLOGICAL | Ph. Eur. | USP |
| Bacterial count | ≤ 10 CFU/100 ml | ≤ 10 CFU/100 ml |
| Bacterial endotoxins | < 0.25 IU/ml | < 0.25 EU/ml |
*data can change without notice

A classic Bram-Cor STMC Vapor Compression still with a customized water pretreatment on the same skid.
Distillation is the main way to produce Water for Injection
There are two basic technologies that have marked the history of the pharmaceutical industry for WFI production: the Vapor Compression (VC) distillation system, by vapor compression stills, and the Multiple Effect (ME) distillation system, by multi stage stills.
In recent years, it has also become possible to obtain cold WFI from Reverse Osmosis. This is due to new generations of membranes that allow the addition of a subsequent ultrafiltration (UF) phase. Below, you can see in a Bram-Cor CROW model (WFI generator via Reverse Osmosis plus Ultrafiltration system) the coupled UF filters (white with yellow-orange heads) downstream positioned.

Producing Water for Injection – Note on pharmacopoeias
USP 27 (2004) revised USP WFI monograph; it admits WFI from “Distillation or a purification process that is equivalent or superior to distillation in the removal of chemicals and microorganisms”. In 2008 EMEA (European Medicines Evaluation Agency, now EMA) issued a Reflection Paper to explain why the use of membrane technology was not considered acceptable for the production of WFI.
From 2010 EDQM (European Directorate for the Quality of Medicines & HealthCare) works on surveys to collect industry data on “Preparation WFI grade water using Reverse Osmosis (RO)”. In 2011 EDQM (Expert Workshop) examines potential uses of the membrane systems for the WFI production and, in 2014, works to allow non-distillation technologies to be included in EP, in addition to distillation for WFI production. In recent years, documents (EMA Q&A EMA / INS / GMP / 489331 / 2016) and related issues are again examined. As a consequence (EP 9.1 publication), HPW monograph (see below) will be deleted.
3. HPW (Highly Purified Water), as defined by Ph.Eur. monograph
(until 2018; no longer defined by Ph. Eur.)
Highly Purified Water (= industrial “Ultrapure” water) is classified by previous Ph.Eur. monograph as an excipient in the production of pharmaceutical products where bacterial endotoxins need to be controlled, unless WFI specified, i.e. for example WFI.
4. WFH (Water For Hemodialysis), as defined by USP monograph.
Water For Hemodialysis is primarily classified in pharmaceutical purposes for hemodialysis applications (see related USP monograph), i.e. for example in the diluition of concentrate solutions. It’s produced from EPA (US Environmental Protection Agency) drinking water which has been further purified to reduce chemical and microbiological components. Note that WFH is not intended for injection.
Note: Purified Water (PW) or Water For Injection (WFI) that are packaged must be sterilized to preserve their microbiological properties; so we have other packaged monographed waters, as Sterile purified water, Sterile water for injection, Bacteriostatic water for injection, and so on (see related USP monographs).

IV Fluids production with Bram-Cor BFIL, linear filling system for bags
B. NON-COMPENDIAL WATERS
Non-compendial waters meet, at least, the requirements of potable water. Note that non-compendial waters are not necessarily of lower quality than compendial waters. But non-compendial water systems may or may not be validated and, generally, non-compendial waters are intended of the appropriate quality required by the single application. For example: Potable Water, Softened water, Ultra Filtration water, Reverse Osmosis Water, EDI (Deionization process) water, Distilled water.

Site sponsored by BRAM-COR Italy | waterforinjection.com Cookie Policy
USEFUL LINKS & SYSTEMS KNOWLEDGE
Main site: bram-cor.com
To view Bram-Cor Equipment catalogue
To view where Bram-Cor Equipment works
About pharmaceutical water treatment systems:
multiple-effect-water-distiller.com
vapor-compression-distiller.com
pharmaceutical-reverse-osmosis-systems.com
pure-steam-generator.com
About pharmaceutical process systems and turnkey plants:
bram-cor-pharmaprocessing.com
ivfluids-parenteralsolutions.com
pharmaceutical-turnkey.com
© 2025 BRAM-COR SPA – VIA MERCALLI 12/A – 43122 PARMA – ITALY – P.IVA 01699350342